Li Ion Battery – How it works
How Li-Ion Batteries Work
Li-ion batteries operate based on electrochemical reactions that enable the storage and release of energy. Inside each battery, there are three main components: the anode (negative electrode), cathode (positive electrode), and electrolyte. During discharge, lithium ions move from the anode to the cathode through the electrolyte, while electrons flow through an external circuit to power devices. This process generates an electric current.
When charging, the process is reversed: lithium ions flow back from the cathode to the anode, and electrons are stored in the anode. A separator within the battery prevents short circuits by keeping the anode and cathode apart while allowing lithium ions to pass through. These batteries are known for their high energy density, rechargeability, and ability to maintain performance over many charge cycles.
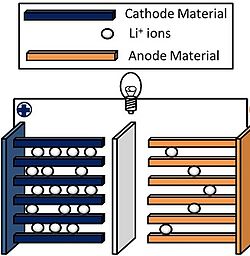
Key Components of Li-Ion Batteries
Li-ion batteries consist of four main components that enable their functionality:
- Cathode: The positive electrode that stores lithium ions and determines the battery's capacity and voltage.
- Anode: The negative electrode where lithium ions are stored during charging; typically made of graphite.
- Electrolyte: A medium that allows lithium ions to move between the cathode and anode while preventing electron flow within the battery.
- Separator: A porous membrane that keeps the cathode and anode apart to prevent short circuits while allowing ion flow.
Discharge and re-charge process
During discharge, lithium ions (Li+Li+) move from the anode (negative electrode) to the cathode (positive electrode) through the electrolyte and separator, while electrons flow externally through the circuit, providing power to the connected device. At the cathode, the lithium ions recombine with electrons and are stored in the cathode's molecular structure, completing the energy release process.
In recharge, an external electrical source applies voltage across the battery, reversing the process. Lithium ions are released from the cathode and migrate back to the anode through the electrolyte. Simultaneously, electrons flow from the cathode to the anode via the external circuit. At the anode, lithium ions and electrons recombine, storing energy chemically in a process called intercalation.
This cycle of ion movement between electrodes is what allows lithium-ion batteries to efficiently store and release energy over multiple charge-discharge cycles.
Li-Ion battery protection
Li-ion batteries are equipped with on-board protection circuits to ensure safe operation and prevent damage during use. The protection system monitors critical parameters such as voltage, current, and temperature to safeguard against potential risks like:
-
Overcharging: Prevents the battery from exceeding its maximum voltage, which could cause overheating or lead to thermal runaway.
-
Over-discharging: Stops the battery from dropping below its minimum voltage, which can result in permanent capacity loss.
-
Short circuits: Detects and interrupts excessive current flow caused by a short circuit, protecting both the battery and connected devices.
These safety features are implemented because Li-ion batteries are highly energy-dense and sensitive to improper handling. The protection circuit ensures reliability, extends battery lifespan, and minimizes risks during charging and discharging.